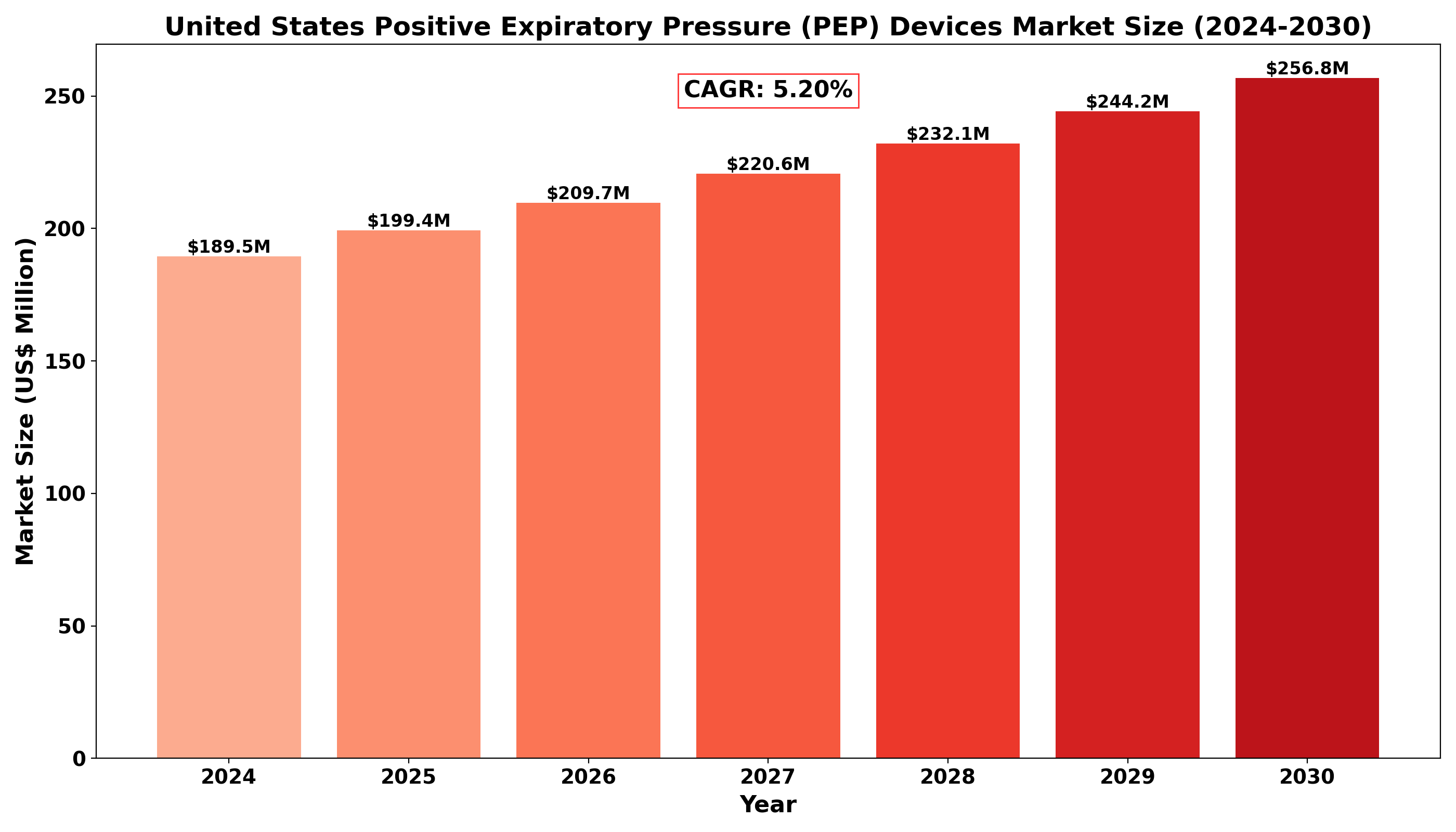
United States Positive Expiratory Pressure (PEP) Devices market was valued at US$ 189.5 million in 2024 and is projected to reach US$ 256.8 million by 2030, at a CAGR of 5.20% during the forecast period 2024-2030.
Respiratory therapy tools that create back pressure when the user exhales, helping to keep airways open and facilitate mucus clearance. Used in the management of various respiratory conditions to improve lung function and reduce the risk of respiratory infections.
This report contains market size and forecasts of Positive Expiratory Pressure (PEP) Devices in United States, including the following market information:
• United States Positive Expiratory Pressure (PEP) Devices Market Revenue, 2019-2024, 2024-2030, ($ millions)
• United States Positive Expiratory Pressure (PEP) Devices Market Sales, 2019-2024, 2024-2030,
• United States Top five Positive Expiratory Pressure (PEP) Devices companies in 2023 (%)
Report Includes
This report presents an overview of United States market for Positive Expiratory Pressure (PEP) Devices , sales, revenue and price. Analyses of the United States market trends, with historic market revenue/sales data for 2019 - 2023, estimates for 2024, and projections of CAGR through 2030.
This report focuses on the Positive Expiratory Pressure (PEP) Devices sales, revenue, market share and industry ranking of main manufacturers, data from 2019 to 2024. Identification of the major stakeholders in the United States Positive Expiratory Pressure (PEP) Devices market, and analysis of their competitive landscape and market positioning based on recent developments and segmental revenues.
This report will help stakeholders to understand the competitive landscape and gain more insights and position their businesses and market strategies in a better way.
This report analyzes the segments data by Type, and by Sales Channels, sales, revenue, and price, from 2019 to 2030. Evaluation and forecast the market size for Humidifier sales, projected growth trends, production technology, sales channels and end-user industry.
• Chronic Obstructive Pulmonary Disease (COPD)
• Asthma
• Atelectasis
• Bronchitis
• Bronchiectasis
• Cystic Fibrosis
• Others
• High Pressure PEP (26-102cm H2O) Devices
• Low Pressure PEP (5-20cm H2O) Devices
Key Companies covered in this report:
• Smiths Medical
• Philips Respironics
• Medtronic plc
• 3M Company
• ResMed
• Hillrom
• Teleflex Incorporated
• Monaghan Medical Corporation
• VyAire Medical
• Allied Healthcare Products Inc.
Fill out the download sample Report form to access the complete list of key players.
The report also provides analysis of leading market participants including:
• Key companies Positive Expiratory Pressure (PEP) Devices revenues in United Statesn market, 2019-2024 (Estimated), ($ millions)
• Key companies Positive Expiratory Pressure (PEP) Devices revenues share in United Statesn market, 2023 (%)
• Key companies Positive Expiratory Pressure (PEP) Devices sales in United Statesn market, 2019-2024 (Estimated),
• Key companies Positive Expiratory Pressure (PEP) Devices sales share in United Statesn market, 2023 (%)
Key Indicators Analysed
• Market Players & Competitor Analysis: The report covers the key players of the industry including Company Profile, Product Specifications, Production Capacity/Sales, Revenue, Price and Gross Margin 2019-2030 & Sales with a thorough analysis of the market’s competitive landscape and detailed information on vendors and comprehensive details of factors that will challenge the growth of major market vendors.
• United Statesn Market Analysis: The report includes United Statesn market status and outlook 2019-2030. Further the report provides break down details about each region & countries covered in the report. Identifying its sales, sales volume & revenue forecast. With detailed analysis by types and applications.
• Market Trends: Market key trends which include Increased Competition and Continuous Innovations.
• Opportunities and Drivers: Identifying the Growing Demands and New Technology
• Porters Five Force Analysis: The report provides with the state of competition in industry depending on five basic forces: threat of new entrants, bargaining power of suppliers, bargaining power of buyers, threat of substitute products or services, and existing industry rivalry.
Key Benefits of This Market Research:
• Industry drivers, restraints, and opportunities covered in the study
• Neutral perspective on the market performance
• Recent industry trends and developments
• Competitive landscape & strategies of key players
• Potential & niche segments and regions exhibiting promising growth covered
• Historical, current, and projected market size, in terms of value
• In-depth analysis of the Positive Expiratory Pressure (PEP) Devices Market
• Overview of the regional outlook of the Positive Expiratory Pressure (PEP) Devices Market
Key Reasons to Buy this Report:
• Access to date statistics compiled by our researchers. These provide you with historical and forecast data, which is analyzed to tell you why your market is set to change
• This enables you to anticipate market changes to remain ahead of your competitors
• You will be able to copy data from the Excel spreadsheet straight into your marketing plans, business presentations or other strategic documents
• The concise analysis, clear graph, and table format will enable you to pinpoint the information you require quickly
• Provision of market value (USD Billion) data for each segment and sub-segment
• Indicates the region and segment that is expected to witness the fastest growth as well as to dominate the market
• Analysis by geography highlighting the consumption of the product/service in the region as well as indicating the factors that are affecting the market within each region
• Competitive landscape which incorporates the market ranking of the major players, along with new service/product launches, partnerships, business expansions, and acquisitions in the past five years of companies profiled
• Extensive company profiles comprising of company overview, company insights, product benchmarking, and SWOT analysis for the major market players
• The current as well as the future market outlook of the industry concerning recent developments which involve growth opportunities and drivers as well as challenges and restraints of both emerging as well as developed regions
• Includes in-depth analysis of the market from various perspectives through Porter’s five forces analysis
• Provides insight into the market through Value Chain
• Market dynamics scenario, along with growth opportunities of the market in the years to come
• 6-month post-sales analyst support
We offer additional regional and global reports that are similar:
• Global Positive Expiratory Pressure (PEP) Devices Market
• United States Positive Expiratory Pressure (PEP) Devices Market
• Japan Positive Expiratory Pressure (PEP) Devices Market
• Germany Positive Expiratory Pressure (PEP) Devices Market
• South Korea Positive Expiratory Pressure (PEP) Devices Market
• Indonesia Positive Expiratory Pressure (PEP) Devices Market
• Brazil Positive Expiratory Pressure (PEP) Devices Market
Customization of the Report: In case of any queries or customization requirements, please connect with our sales team, who will ensure that your requirements are meet.
1.1 Positive Expiratory Pressure (PEP) Devices Product Introduction
1.2.1 United Statesn Positive Expiratory Pressure (PEP) Devices Market Size Growth Rate by Type, 2019 VS 2023 VS 2030
1.2.2 Chronic Obstructive Pulmonary Disease (COPD)
1.2.3 Asthma
1.2.4 Atelectasis
1.2.5 Bronchitis
1.2.6 Bronchiectasis
1.2.7 Cystic Fibrosis
1.2.8 Others
1.3.1 United States Positive Expiratory Pressure (PEP) Devices Market Size Growth Rate by Application, 2019 VS 2023 VS 2030
1.3.2 High Pressure PEP (26-102cm H2O) Devices
1.3.3 Low Pressure PEP (5-20cm H2O) Devices
1.4 United States Positive Expiratory Pressure (PEP) Devices Sales Estimates and Forecasts 2019-2030
1.5 United States Positive Expiratory Pressure (PEP) Devices Hydrocephalus Shunts Revenue Estimates and Forecasts 2019-2030
1.6 Study Objectives
1.7 Years Considered
2.1 United States Positive Expiratory Pressure (PEP) Devices Sales by Manufacturers
2.1.1 United States Positive Expiratory Pressure (PEP) Devices Sales by Manufacturers (2019-2024)
2.1.2 United States Positive Expiratory Pressure (PEP) Devices Sales Market Share by Manufacturers (2019-2024)
2.1.3 Top Largest Manufacturers of Positive Expiratory Pressure (PEP) Devices in 2023 in United States
2.2 United States Positive Expiratory Pressure (PEP) Devices Revenue by Manufacturers
2.2.1 United States Positive Expiratory Pressure (PEP) Devices Revenue by Manufacturers (2019-2024)
2.2.2 United States Positive Expiratory Pressure (PEP) Devices Revenue Market Share by Manufacturers (2019-2024)
2.2.3 United States Top Companies by Positive Expiratory Pressure (PEP) Devices Revenue in 2023
2.3 United States Positive Expiratory Pressure (PEP) Devices Sales Price by Manufacturers (2019-2024)
2.4 Analysis of Competitive Landscape
2.4.1 Manufacturers Market Concentration Ratio (CR3 and HHI)
2.4.2 United States Positive Expiratory Pressure (PEP) Devices by Company Type (Tier 1, Tier 2, and Tier 3)
2.4.3 United States Positive Expiratory Pressure (PEP) Devices Manufacturers Geographical Distribution
2.5 Mergers & Acquisitions, Expansion Plans
3.1 Positive Expiratory Pressure (PEP) Devices Market Size by Region: 2019-2030
3.1.1 United States Positive Expiratory Pressure (PEP) Devices Sales by Region: 2019-2024
3.1.2 United States Positive Expiratory Pressure (PEP) Devices Sales Forecast by Region (2025-2030)
3.1.3 United States Positive Expiratory Pressure (PEP) Devices Revenue by Region: 2019-2024
3.1.4 United States Positive Expiratory Pressure (PEP) Devices Revenue Forecast by Region (2025-2030)
4.1 United States Positive Expiratory Pressure (PEP) Devices Sales by Type
4.1.1 United States Positive Expiratory Pressure (PEP) Devices Historical Sales by Type (2019-2024)
4.1.2 United States Positive Expiratory Pressure (PEP) Devices Forecasted Sales by Type (2025-2030)
4.1.3 United States Positive Expiratory Pressure (PEP) Devices Sales Market Share by Type (2019-2030)
4.2 United States Positive Expiratory Pressure (PEP) Devices Revenue by Type
4.2.1 United States Positive Expiratory Pressure (PEP) Devices Historical Revenue by Type (2019-2024)
4.2.2 United States Positive Expiratory Pressure (PEP) Devices Forecasted Revenue by Type (2025-2030)
4.2.3 United States Positive Expiratory Pressure (PEP) Devices Revenue Market Share by Type (2019-2030)
4.3 United States Positive Expiratory Pressure (PEP) Devices Price by Type
4.3.1 United States Positive Expiratory Pressure (PEP) Devices Price by Type (2019-2024)
4.3.2 United States Positive Expiratory Pressure (PEP) Devices Price Forecast by Type (2025-2030)
5.1 United States Positive Expiratory Pressure (PEP) Devices Sales by Application
5.1.1 United States Positive Expiratory Pressure (PEP) Devices Historical Sales by Application (2019-2024)
5.1.2 United States Positive Expiratory Pressure (PEP) Devices Forecasted Sales by Application (2025-2030)
5.1.3 United States Positive Expiratory Pressure (PEP) Devices Sales Market Share by Application (2019-2030)
5.2 United States Positive Expiratory Pressure (PEP) Devices Revenue by Application
5.2.1 United States Positive Expiratory Pressure (PEP) Devices Historical Revenue by Application (2019-2024)
5.2.2 United States Positive Expiratory Pressure (PEP) Devices Forecasted Revenue by Application (2025-2030)
5.2.3 United States Positive Expiratory Pressure (PEP) Devices Revenue Market Share by Application (2019-2030)
5.3 United States Positive Expiratory Pressure (PEP) Devices Price by Application
5.3.1 United States Positive Expiratory Pressure (PEP) Devices Price by Application (2019-2024)
5.3.2 United States Positive Expiratory Pressure (PEP) Devices Price Forecast by Application (2025-2030)
6.1 Smiths Medical
6.1.1 Smiths Medical Corporation Information
6.1.2 Smiths Medical Overview
6.1.3 Smiths Medical in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.1.4 Smiths Medical Positive Expiratory Pressure (PEP) Devices Product Introduction
6.1.5 Smiths Medical Recent Developments
6.2 Philips Respironics
6.2.1 Philips Respironics Corporation Information
6.2.2 Philips Respironics Overview
6.2.3 Philips Respironics in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.2.4 Philips Respironics Positive Expiratory Pressure (PEP) Devices Product Introduction
6.2.5 Philips Respironics Recent Developments
6.3 Medtronic plc
6.3.1 Medtronic plc Corporation Information
6.3.2 Medtronic plc Overview
6.3.3 Medtronic plc in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.3.4 Medtronic plc Positive Expiratory Pressure (PEP) Devices Product Introduction
6.3.5 Medtronic plc Recent Developments
6.4 3M Company
6.4.1 3M Company Corporation Information
6.4.2 3M Company Overview
6.4.3 3M Company in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.4.4 3M Company Positive Expiratory Pressure (PEP) Devices Product Introduction
6.4.5 3M Company Recent Developments
6.5 ResMed
6.5.1 ResMed Corporation Information
6.5.2 ResMed Overview
6.5.3 ResMed in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.5.4 ResMed Positive Expiratory Pressure (PEP) Devices Product Introduction
6.5.5 ResMed Recent Developments
6.6 Hillrom
6.6.1 Hillrom Corporation Information
6.6.2 Hillrom Overview
6.6.3 Hillrom in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.6.4 Hillrom Positive Expiratory Pressure (PEP) Devices Product Introduction
6.6.5 Hillrom Recent Developments
6.7 Teleflex Incorporated
6.7.1 Teleflex Incorporated Corporation Information
6.7.2 Teleflex Incorporated Overview
6.7.3 Teleflex Incorporated in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.7.4 Teleflex Incorporated Positive Expiratory Pressure (PEP) Devices Product Introduction
6.7.5 Teleflex Incorporated Recent Developments
6.8 Monaghan Medical Corporation
6.8.1 Monaghan Medical Corporation Corporation Information
6.8.2 Monaghan Medical Corporation Overview
6.8.3 Monaghan Medical Corporation in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.8.4 Monaghan Medical Corporation Positive Expiratory Pressure (PEP) Devices Product Introduction
6.8.5 Monaghan Medical Corporation Recent Developments
6.9 VyAire Medical
6.9.1 VyAire Medical Corporation Information
6.9.2 VyAire Medical Overview
6.9.3 VyAire Medical in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.9.4VyAire Medical Positive Expiratory Pressure (PEP) Devices Product Introduction
6.9.5 VyAire Medical Recent Developments
6.10 Allied Healthcare Products Inc.
6.10.1 Allied Healthcare Products Inc. Corporation Information
6.10.2 Allied Healthcare Products Inc. Overview
6.10.3 Allied Healthcare Products Inc. in United States: Positive Expiratory Pressure (PEP) Devices Sales, Price, Revenue and Gross Margin (2019-2024)
6.10.4 Allied Healthcare Products Inc. Positive Expiratory Pressure (PEP) Devices Product Introduction
6.10.5 Allied Healthcare Products Inc. Recent Developments
7.1 Positive Expiratory Pressure (PEP) Devices Industry Chain Analysis
7.2 Positive Expiratory Pressure (PEP) Devices Key Raw Materials
7.2.1 Key Raw Materials
7.2.2 Raw Materials Key Suppliers
7.3 Positive Expiratory Pressure (PEP) Devices Production Mode & Process
7.4 Positive Expiratory Pressure (PEP) Devices Sales and Marketing
7.4.1 Positive Expiratory Pressure (PEP) Devices Sales Channels
7.4.2 Positive Expiratory Pressure (PEP) Devices Distributors
7.5 Positive Expiratory Pressure (PEP) Devices Customers
8.1.1 Positive Expiratory Pressure (PEP) Devices Industry Trends
8.1.2 Positive Expiratory Pressure (PEP) Devices Market Drivers
8.1.3 Positive Expiratory Pressure (PEP) Devices Market Challenges
8.1.4 Positive Expiratory Pressure (PEP) Devices Market Restraints
10.1 Research Methodology
10.1.1 Methodology/Research Approach
10.1.2 Data Source
10.2 Author Details
10.3 Disclaimer
Frequently Asked Questions ?
